Bohr's Atomic Model Differed From Rutherford's Because It Explained That
Rutherfords model explained the physical and chemical properties of elements whereas Bohrs model only explained the. Buhrs atomic model differed from ruthofords because it explained that.
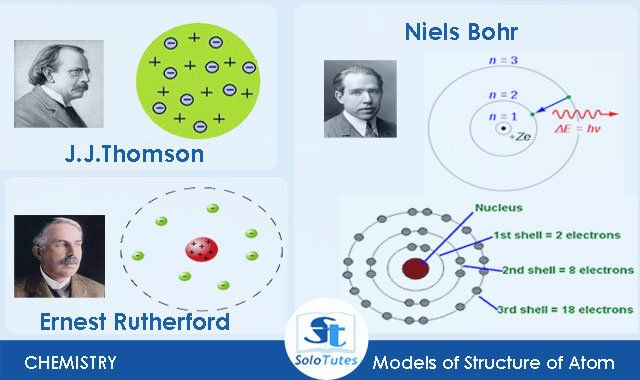
Thomson Rutherford And Bohr Model Of Atom
Electrons are embedded within the rest of the atom like plum pudding.
. In 1915 Neil Bohr proposed the Bohr model of the atom. Bohrs Theory Bohrs Atomic Model. 7 rows Niels Bohr postulates the atomic model which states that electrons move in specific circular.
Rutherford proposed that most of the atom was empty space with the mass and positive charge concentrated in a tiny nucleus. Bohrs model consists of a small nucleus positively charged surrounded by negative. It was created after Rutherfords atomic model was modified.
Rutherford model and Bohr model are models that explain the structure of an atom. Rutherfords atomic model was based on a central nucleus surrounded by. In the year 1913 Niels Bohr proposed an atomic structure model describing an atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits around the positively charged.
Electrons would orbit the nucleus similar to how planets orbit the sun. An atom is an. Dalton s atomic model thomson s atomic model rutherford s atomic model bohr s atomic model quantum atomic model.
Thomsons atomic model and Rutherfords atomic model failed to answer any questions related to the energy of an atom and its stability. Rutherfords model introduced the nuclear model of an atom describing how a positively charged nucleus is surrounded by negatively charged electrons. Rutherford model was proposed by Ernest Rutherford in 1911.
Rutherfords model showed that the positive charge is concentrated in a small area. Niels Bohr proposed a theory called the Bohr theory. Bohr model is considered as a modification of Rutherford model.
Bohr model was proposed by Niels Bohr in 1915. The main difference between Rutherford and Bohr model is that Rutherford model does not explain the. Bohr proposed an atomic.
He believed that electrons moved around the nucleus in. How Does Bohrs Model Of The Atom Differ From RutherfordsRutherford described the atom as consisting of a tiny positive mass surrounded by a cloud of negative electrons. Four models of the atom are shown below but one important model is missing.
Rutherfords model proved that electrons are dispersed in a cloud of positive. Rutherfords model showed that electrons orbit the nucleus with distinct radii. Bohrs atomic model differed from Rutherfords because it explained that The model of the atom has changed as scientists have gathered new evidence.
Bohr thought that electrons orbited the nucleus in quantised orbits. Rutherford basically explained the nucleus of an atom and Bohr modified that model into electrons and their energy levels. Social Studies 19072019 0830.
Bohrs atomic model differed from Rutherfords because it explained that electrons exist in specified energy levels surrounding the nucleus option A Explanation. Answer 1 of 2. Bohrs atomic model differed from Rutherfords because it explained that electrons exist in specified energy levels surrounding the nucleus.
How did rutherfords atomic model fix the shortcomings of thomsons atomic model. Rutherfords model identified that the electrons were at a distance from the nucleus Bohrs model identified that the electrons occurred at levels that related to their available energy and the modern atomic model shows that electrons are located in a predicted area but cannot be identified in a specific point. Bohrs model states that electrons occupy orbitals with fixed energy levels.
Bohrs Model of an Atom. However Rutherfords model did not explain why the negative orbiting electron did not lose energy and be pulled into the positive nucleus. Heres your answer Rutherford did discovered the nucleus and electrons moving around the nucleus but one limitation in his model of atom was that if the electrons are moving around the nucleus radiating some energy.
Questions in other subjects. How did Bohrs model differ from Rutherfords quizlet.
In What Ways Does The Bohr Model Of Atoms Better Explain Nature Than Rutherford S Model Quora
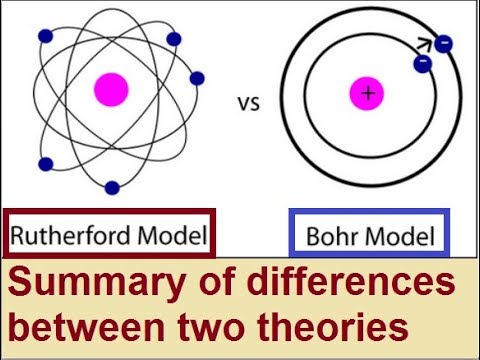
7 Difference Between Bohr And Rutherford Model With Explanation Core Differences
Difference Between Rutherford And Bohr Model Definition Explanation Of The Models
Comments
Post a Comment